RSV Vaccine - New Rules
The June ACIP meeting brought new rules about which older people should get RSV vaccine. And a new mRNA RSV vaccine from Moderna that had no GBS signal.
RSV Burden
"In the United States, respiratory syncytial virus (RSV) infections cause an estimated 58,000–80,000 hospitalizations among children aged <5 years and 60,000–160,000 hospitalizations among adults aged ≥65 years each year. U.S. RSV epidemics typically follow seasonal patterns, peaking in December or January." - from https://www.cdc.gov/mmwr/volumes/72/wr/mm7214a1.htm
RSV Vaccine Decision Process
The Advisory Committee on Immunization Practices (ACIP) meets at least thrice yearly at the CDC offices in Atlanta. It reviews FDA-approved vaccine evidence and issues vaccine recommendations, which become the official U.S. vaccine recommendations once reviewed and approved by the CDC Director and Department of Health and Human Services. Their role is detailed here.
Maternal Recommendations Unchanged
CDC recommends a respiratory syncytial virus (RSV) vaccine for pregnant people to protect their babies from severe RSV disease. Pregnant people should get a single dose of Pfizer’s bivalent RSVpreF vaccine (Abrysvo) during weeks 32 through 36 of pregnancy during September through January.
To prevent severe RSV disease in infants, either maternal RSV vaccination or infant immunization with RSV monoclonal antibody is recommended. Most infants will not need both.
Full details on the maternal recommendations are here.
Older Adult Recommendation Changed
The recommendations for the RSV vaccine in older adults were revised at the ACIP June 2024 meeting. The new CDC recommendation is a single dose of RSV vaccine for:
- All adults ages 75 and older
- Adults ages 60-74 who are at increased risk of severe RSV disease
Full details, including what conditions qualify for increased risk of severe RSV disease, are here.
2023 RSV Vaccines
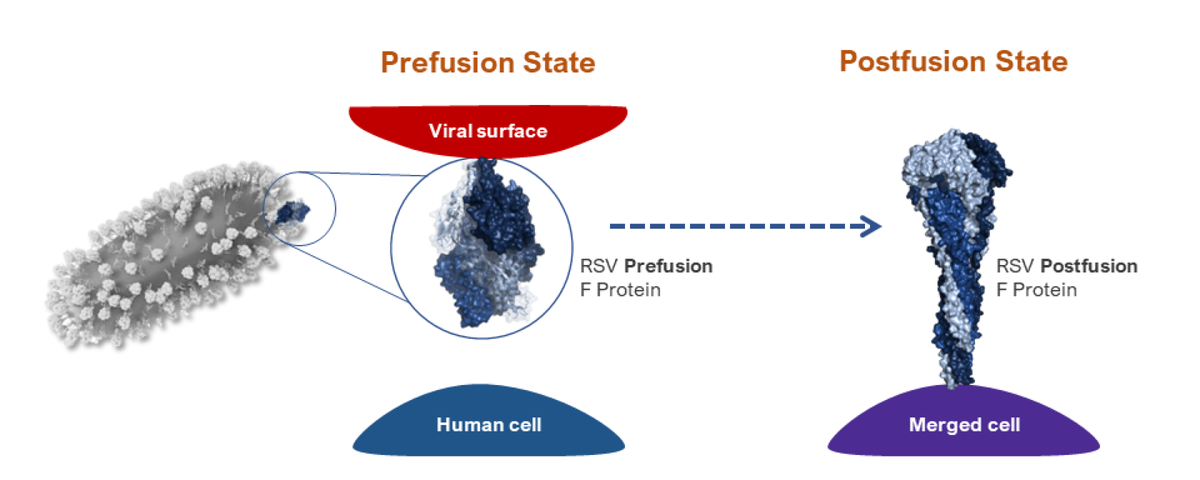
Two RSV vaccines were approved in 2023: Abryso (Pfizer) and Arexvy (GSK). Both were thought to be effective for at least two RSV seasons. Only Abryso was tested in pregnant women, so it is the only vaccine approved for that population. Both were approved for older adults. Both vaccines are the traditional vaccine type with stabilized prefusion F protein (see caption of the image above).
These vaccine prefusion F proteins are locked into a prefusion state and, when injected into us, are seen as foreign. Thus, they trigger the immune response to destroy them and give us a memory to eliminate the RSV virus (which has prefusion F protein on its membrane) when we later get exposed to it. Arexvy uses an adjuvant in its vaccine. The CDC concluded that both were equally effective, and it does not matter which one an older adult uses.
Guillain-Barré Syndrome (GBS) concern
In 2023, the concern was that clinical trials for both vaccines had a signal for GBS or similar syndromes that was higher than the average amount of GBS expected in the population. There were three cases in each of the vaccine trials. Because of this, the 2023 recommendation was that older adults should only receive the vaccine after sharing decision-making with their physician.
CDC has a page here that details how they evaluate GBS and vaccines. A 2011 systematic review and meta-analysis found:
Of 1,683 nonduplicate citations, 16 met the inclusion criteria, which produced 1,643 cases and 152.7 million person-years of follow-up. GBS incidence increased by 20% for every 10-year increase in age; the risk of GBS was higher for males than females. The regression equation for calculating the average GBS rate per 100,000 person-years as a function of age in years was exp[−12.0771 + 0.01813(age in years)] × 100,000.
The above numbers give a background GBS incidence rate of 10.8 per million. The background GBS rate will be higher if the population is older. To determine if there is a statistically higher rate of GBS after vaccination, one must see if the actual rate after vaccination is higher than the expected background rate adjusted for the age of the patients receiving the vaccination enrolled in the follow-up study.
2023 RSV Vaccine Follow-up Study
A follow-up surveillance study of those receiving the 2023 vaccines (detailed here) showed no statistical signal for GBS, though there were cases recorded.
FDA did their follow-up study of GBS after RSV vaccination, detailed here, and concluded:
- The results from two different types of analyses of potential GBS risk
following RSV vaccination are mixed and highly uncertain - These analyses do not provide clear, conclusive evidence of an elevated
risk of GBS and an elevated risk cannot be ruled out at this time - FDA is conducting medical chart review on GBS cases and will continue to
evaluate the safety of RSV vaccines as more data are available - FDA maintains that the benefits of RSV vaccination in preventing RSV
hospitalizations outweigh the potential risks associated with the vaccines
New 2024 mRNA Vaccine From Moderna
Modern's mRNA RSV vaccine was approved by the FDA on 5/31/2024, and based on the ACIP June 2024 meeting and CDC approval, it is now one of the RSV vaccines recommended for those who should get the RSV vaccine. The official CDC recommendation is that it does not matter which of the three approved RSV vaccines one receives as an older adult.
The mRNA vaccine mechanism also involves using a prefusion F protein locked into the pre-fusion state. But instead of injecting the prefusion F protein into us, it injects an mRNA complex that goes into our cells and programs our cells' ribosome factories to produce the F protein locked into the prefusion state, which then gets transported out of the cells into the bloodstream, thus triggering our immune response.
The Moderna mRNA RSV vaccine did not have any GBS signal or cases in their clinical trial. The placebo-controlled clinical trial was conducted with 18,427 participants who were 60 years of age or older. Full details are here.
Conclusion
The national recommendation is to get an RSV vaccine if you are in one of the groups that should get it. As confirmed by the FDA, ACIP, and CDC, the benefits outweigh the risks for those groups. Getting the RSV vaccine at this time is not recommended unless you are in one of the specified groups.
Which RSV vaccine should you get if you are an older adult? The official CDC recommendation is that it does not matter. Just get the one that is available to you. As there is still some uncertainty about GBS risk in the 2023 vaccines, some may wish to choose the Moderna mRNA RSV vaccine, which had no GBS cases in their clinical trial.